
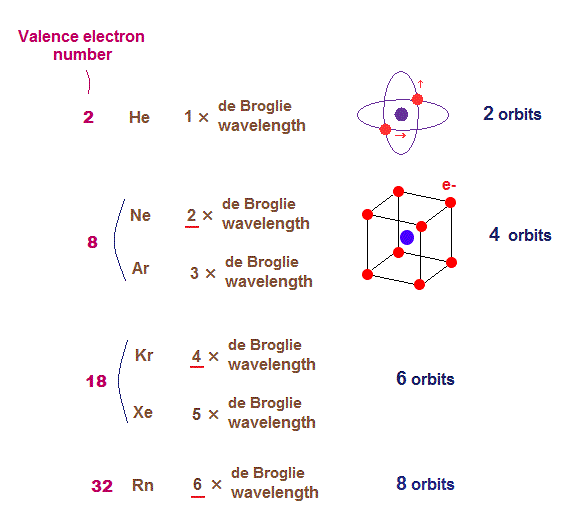
Does Xe have 18 valence electrons?Īnswer and Explanation: Xenon has eight valence electrons, which are the electrons in its outer shell. Thus, krypton is neither a compound or a mixture. It only consists of 1 type of atom and it cannot be broken down to produce other elements. It’s a noble gas with the atomic number of 36. Is krypton an element compound or mixture? Air is also the most important source for the other noble gases, with the exception of helium (obtained from natural gas) and radon (obtained as a byproduct of the decay of radioactive elements). How is krypton in nature?Īlthough traces of krypton are found in various minerals, the most important source of krypton is Earth’s atmosphere. See also What is SpaceX stock code? How many isotopes does krypton have?Ī total of 32 isotopes of krypton have been identified, having atomic masses ranging from 69 to 100. This becomes relevant in nuclear chemistry, where sometimes, you have to balance nuclear equations with respect to the mass numbers (nucleon numbers). In the case of Krypton-84, this means that you have 84 nucleons, where 36 of these are protons, and the remaining 48 are neutrons. Noble gases have a complete octet therefore, they are. Krypton-86 is composed of 36 protons, 50 neutrons, and 32 electrons. The largest amount is found in the noble gases (group 18), which have a total of eight valence electrons. Krypton-84 is composed of 36 protons, 48 neutrons, and 32 electrons. Since the last shell(orbit) of a gallium ion has eighteen electrons, the valence electrons of gallium ions(Ga3+) are eighteen. How many valence electrons does GA 3 have? So first step, we just have to account for the valence electrons.
How many valence electrons are there in Xe? About three times heavier than air, krypton is colourless, odourless, tasteless, and monatomic. Krypton (Kr), chemical element, a rare gas of Group 18 (noble gases) of the periodic table, which forms relatively few chemical compounds. Moreover, we discuss the easiest method of. Krypton is a noble gas in group 18, period 4, and the p-block of the periodic table. How many valence electrons does krypton have (A) 36 (B) 6 (C) 8 (D) 4 (E) 10. The stability of an element’s outer (valence) electrons determines its chemical and physical properties. How many shells does Krypton have?ģ6 electrons (white) occupy shells (rings) eight fill the outer (fourth) electron shell in what is a very stable configuration. There are 48 neutrons inside the nucleus of one atom of Krypton. The atomic mass number of Krypton is 83.80 or 84 and the atomic number is 36, so, 84-36=48. See also Are King Palms better for you? What is the neutrons of krypton? How many neutrons and electrons are in krypton? Krypton-78 is composed of 36 protons, 42 neutrons, and 36 electrons. How many protons and electrons does krypton have? Gallium therefore has three valence electrons. The 4s and 4p electrons can be lost in a chemical reaction, but not the electrons in the filled 3d subshell. Gallium has the following electron configuration. So to find the valence electrons, one would look at the highest principal quantum number for whats written out, and for silver thats 5, so in Kr 4d10 5s1. There are equal numbers of valence electrons in a group.
Krypton number of valence electrons full#
The other inert gases including argon and xenon also have full outer shells with eight electrons. Valence electrons across a period are in the same energy level. This problem has been solved See the answer. This is one of the happy elements and has an electron configuration of 2-8-18-8. How many valence electrons are in the Lewis-dot (electron dot) structure for the neutral krypton (Kr) atom 03 O 6. Since krypton is in the far right row of the periodic table, its outermost shell is full with eight electrons. How many core and valence electrons does krypton have?
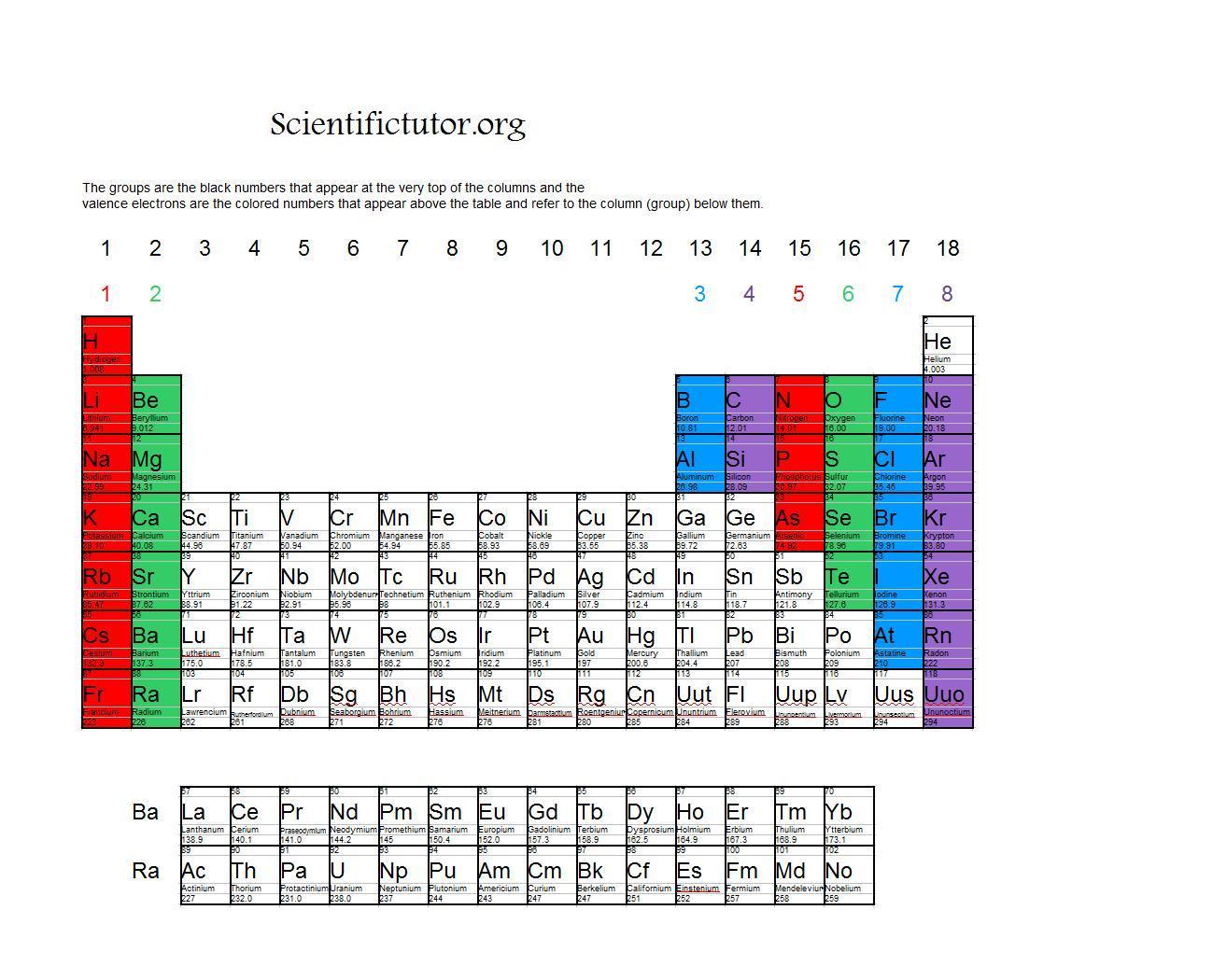
How many core and valence electrons does krypton have?.All elements can be represented in this fashion.


 0 kommentar(er)
0 kommentar(er)
